 The relationship between the contents, metabolites, barrier and immune response of the gut and organs and function in the body are becoming well understood, albeit there are many nuances yet to be quantified.
The relationship between the contents, metabolites, barrier and immune response of the gut and organs and function in the body are becoming well understood, albeit there are many nuances yet to be quantified.
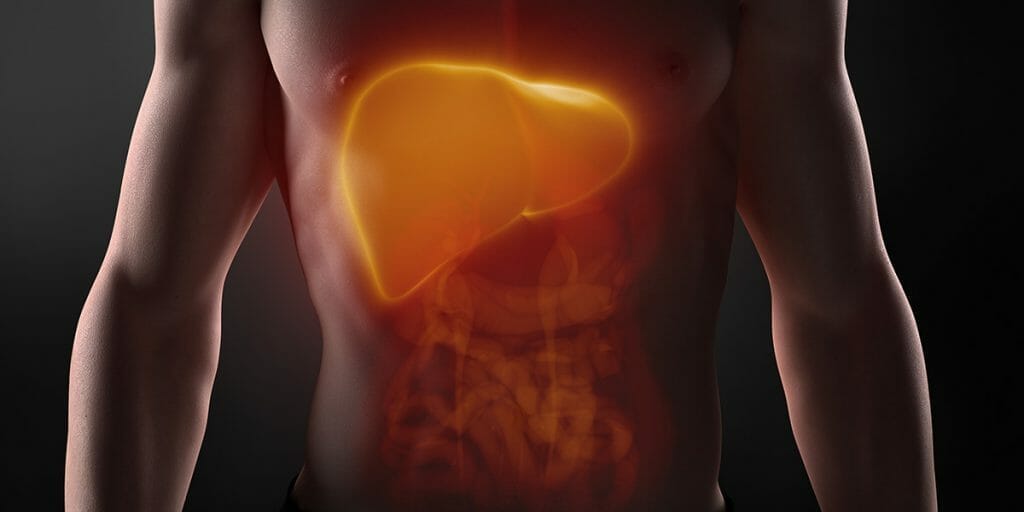
One area in which the dynamic interaction between the gut and organs is rapidly rising up the knowledge tree is the ‘liver and gut axis’. In large part this is due to the increase in the prevalence of liver related inflammation, of which non-alcoholic fatty liver disease (NAFLD) is becoming a global problem.
The spectrum of NAFLD ranges from simple hepatic steatosis, commonly associated with obesity, to non-alcoholic steatohepatitis, which can progress to fibrosis, cirrhosis and hepatocellular carcinoma. NAFLD pathophysiology is understood to involve a mix of environmental, physical, genetic and metabolic factors, as well as changes in the intestinal microbiota and their products.
The term ‘gut-liver axis’ is not new, having been first described by Volta et al in 1978, when these researchers reported production of immunoglobulin A antibodies to dietary antigens in patients with liver cirrhosis indicating interactions between gut and liver.[1]
The liver is unique in its supply of blood in that it has two sources; from the hepatic artery and portal vein. Some 70%–75% of the liver’s blood supply is derived from the portal vein, which drains blood from mesenteric veins of the intestinal tract. This means that nutrients such as monosaccharides and amino acids are absorbed by specialised transporters on enterocytes and can reach the liver through the portal vein, where many are taken up by the liver cells – hepatocytes and then metabolised. If the gut barrier is disrupted and contents entering the submucosa are problematic, the liver is the first organ in the body that encounters these including microbial products, toxins and microorganisms (such as bacteria and fungi) from the intestine. The liver therefore serves as a large collection base for compounds and substances originating from the intestine and as such has to respond.
 Any related barrier dysfunction also adds to the metabolic challenge as gut barrier dysfunction results in translocation of microbes, their secreted metabolites and other microbial products from the gut lumen into blood and lymphatics, allowing them to reach other tissues and organs including the liver. Not all people with fatty livers have increased permeability but studies suggest about 40% do, suggesting its role in the development of NAFLD is not essential but clinically relevant.[2] The metabolic components linked to altered bacterial composition and diet derived substrates are increasingly seen as being instrumental in the generation and progression of NAFLD. Microbe-derived metabolites, such as trimethylamine, some secondary bile acids, short-chain fatty acids and ethanol appear likely to contribute to the pathogenesis of NAFLD.[3]
Any related barrier dysfunction also adds to the metabolic challenge as gut barrier dysfunction results in translocation of microbes, their secreted metabolites and other microbial products from the gut lumen into blood and lymphatics, allowing them to reach other tissues and organs including the liver. Not all people with fatty livers have increased permeability but studies suggest about 40% do, suggesting its role in the development of NAFLD is not essential but clinically relevant.[2] The metabolic components linked to altered bacterial composition and diet derived substrates are increasingly seen as being instrumental in the generation and progression of NAFLD. Microbe-derived metabolites, such as trimethylamine, some secondary bile acids, short-chain fatty acids and ethanol appear likely to contribute to the pathogenesis of NAFLD.[3]
Hence changing the metabolic output from the disordered microbiota is a clinically useful adjunctive intervention along with other strategic lifestyle interventions and supplements to prevent or reverse NAFLD. Gut microbiota are increasingly regarded as a novel therapeutic target for NAFLD by manipulating it in various ways, including probiotics, prebiotics, synbiotics, antibiotics, faecal microbiota transplantation, and herbal components.[4] Probiotics studied to date include lactic acid and bifido bacteria in which strain specific bacteria have been tested, however, whilst improvements in numerous parameters are recorded there is inconsistency – suggesting that the use of a probiotic alone is unlikely to be adequate and that diet and exercise changes as well as specific prebiotic foods will be needed.
References:
- Volta U, Bonazzi C, Bianchi FB, et al. IgA antibodies to dietary antigens in liver cirrhosis. Ric Clin Lab 1987;17:235–42. View Abstract
- Luther J, Garber JJ, Khalili H, Dave M, Bale SS, Jindal R, Motola DL, Luther S, Bohr S, Jeoung SW, Deshpande V, Singh G, Turner JR, Yarmush ML, Chung RT, Patel SJ. Hepatic Injury in Nonalcoholic Steatohepatitis Contributes to Altered Intestinal Permeability. Cell Mol Gastroenterol Hepatol. 2015 Mar;1(2):222-232. View Full Paper
- Chu H, Duan Y, Yang L, Schnabl B. Small metabolites, possible big changes: a microbiota-centred view of non-alcoholic fatty liver disease. Gut. 2018 Aug 31 View Abstract
- Han R, Ma J, Li H. Mechanistic and therapeutic advances in non-alcoholic fatty liver disease by targeting the gut microbiota. Front Med. 2018 Sep 4. View Abstract
